Generic Remdesivir in India
US drugmaker Gilead Sciences said it had signed non-exclusive voluntary licensing agreements with five generic pharmaceutical manufacturers, including three based in India, to further expand the supply of antiviral drug Remdesivir to treat COVID-19 like it did with hepatitis C drug in 2014.
The agreements allow the companies – Cipla, Hetero Labs, Jubilant Lifesciences, Mylan and Pakistan-based Ferozsons Laboratories, to manufacture Remdesivir for distribution in 127 countries.
Under the licensing agreements, the companies have the right to receive a technology transfer of the Gilead manufacturing process for Remdesivir to enable them to scale up production more quickly.
The licensees also set their own prices for the generic product they produce. The licenses are royalty-free until the World Health Organization (WHO) declares the end of the Public Health Emergency of International Concern regarding COVID-19, or until a pharmaceutical product other than Remdesivir or a vaccine is approved to treat or prevent COVID-19, whichever is earlier.
Indian pharma company Hetero Labs is the first company to launch covifor generic Remdesivir in India. Hetero launch generic Remdesivir with the brand name Covifor and Cipla Ltd launched with the name of Cipremi in India.
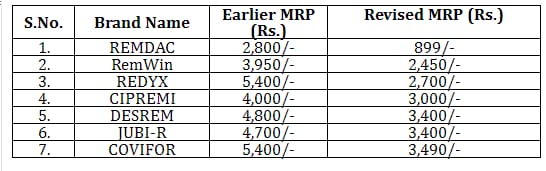
Price of Generic Remdesivir in India
Cipla’s version is priced at less than 5,000 Indian rupees (around $67), while Hetero Lab’s version is priced at 5,400 rupees (around $71).
Mylan prices its DESREM (generic Remdesivir) in India at $64 per 100 mg vial
As per the report, Gilead Prices COVID-19 drug Remdesivir at $2,340 per patient in developed wealthier nations.
Generic Remdesivir price in India about 80% below the price tag on the drug for wealthy nations.
Bangladesh Beximco Pharmaceuticals Ltd. said it has become the world’s first company to start selling the generic version of Remdesivir drug to treat the infection caused by the deadly coronavirus across the developing world. Beximco sell the drug under the brand name bemsivir
Updated on 21st April 2021
As per the second wave of Covid 19 In India, the Indian government has reduced the prices of Remdesivir.
In the second wave of Covid-19 in India Remdesivir demand is suddenly increased due to high demand, Indian pharmaceuticals companies started more productions of Remdesivir to fulfil the demand.
Remdesvir is not required for every Covid patient, it is only for the critical patient. Remdesivir stops further multiplication of Covid virus. In this case the chances of patient recovery increase.
Zydus Cadila manufacturing the lowest cost Remdesivir in India with the brand name REMDAC. The cost of REMDAC (Remdesivir) one vial injuctin is INR 899/- now.
Remdesivir
Currently, no drugs or vaccines have been developed to completely cure coronavirus. But some medicines are being said to be used in emergency situations, this mainly includes the name of the drug Remdesivir, made by the American company Gilead Sciences.
Remdesivir is a broad-spectrum antiviral drug. It was originally developed to treat Ebola, another infectious disease but had only limited success.
In February 2020, Chinese scientists found that Remdesivir successfully blocked the novel coronavirus, SARS-CoV-2, from replicating in human cells.
Remdesivir drug directly attacks the virus. Remdesivir prevents the virus from replicating, thereby stopping the growth of the virus in the body. It is the injection form.
According to reports, It has been approved by the United States Food and Drug Administration (USFDA) to use the drug Remdesivir in the treatment of corona patients.
There have been some good results from the use of this drug in the US, which is being advised to be used in emergency situations in the US.
Japan has also given regulatory approval to the company on the basis of USFDA approval.
At the same time, now this company has applied to the Central Pharmaceutical Standards Control Organization (CDSCO) of India for permission to sell the drug in the Indian market.
India’s drug regulator has granted US pharma giant Gilead Sciences marketing authorization for its anti-viral drug Remdesivir for “restricted emergency use” on hospitalised COVID-19 patients in view of the crisis posed by the pandemic.
With the formation of the coronavirus vaccine, the world’s hopes are also set on medicines proving effective in its treatment. For the time being, Remdesivir is the only drug that works in the treatment of Covid 19, which is very effective in the treatment of corona.
This drug is also among the four medicines that the World Health Organization is testing under the Solidarity Trial.
Clinical study
Data related to Remdesivir, the first and only drug to benefit patients with coronavirus, have been published for the first time. After about a month of trial, scientists working with the US government have published a study related to medicine. Doctors have been demanding data related to medicine for a long time.
According to nytimes.com report, scientists have claimed that the drug Remdesivir benefits critically ill corona patients.
According to Reuters, the US National Institutes of Health says that the trial of Remdesivir showed that this drug provides the most benefit to patients who need only oxygen, but no ventilators.
At the same time, a study sponsored by the US National Institute of Allergy and Infectious Diseases was published on Friday evening on the website of The New England Journal of Medicine. The study claimed that the drug Remdesivir succeeded in reducing the recovery time of corona patients from 15 days to 11 days.
The study said that 1063 serious patients were given Remdesivir medicine or placebo. Those who were given Remdesivir medicine saw greater improvement than placebo. Actually, placebo is like a real medicine but inactive Substance is used in it.
Dr. Andrey Kalil said that all types of patients, including whites, blacks and Latin Americans, benefited equally from the drug. Not only this, equal gains were also seen in men and women.
According to the Phase Three clinical trial, the condition of about 65 percent of patients has been seen to improve significantly on the 11th day after eating this medicine for five consecutive days.
Special permission is required from the doctor to use this medicine on patients. Remadecivir will only be used in the hospital.
Indian generic medicine
Drug patents are an important issue in India as many countries depend on countries manufacturing generic drugs for cheaper versions of essential medicines. Generic medicines are mass-produced in India. Gilead has three patents in India regarding the drug Remdasivir. It is from 2009 when this medicine started to be used to treat Ebola.
India has no match in the world of generic drugs and vaccines. The demand for hydroxychloroquine during the COVID-19 crisis has proved that India has emerged as the world’s biggest hope in the world of medicines. India is exporting generic drugs to many countries of the world.
This is how India became the leader in the world of medicines:
Indian companies launched second legally valid versions of reputed drugs through reverse engineering. In 1995, the World Trade Organization (WTO) introduced an agreement to give 20 years of protection to drug patents and gave companies 10 years to comply.
However, when the HIV crisis came, it was clear that poor countries needed cheaper medicines. The WTO considered that member countries could license manufacturers to make generic versions of medicines necessary to protect public health. In 2001, Indian pharmaceutical company Cipla did reverse engineering of many brand name drugs. The drug was offered for one dollar a day to African countries and aid groups, making it more than 96 percent cheaper than the brand-name versions.
Low Price, Similar Results
Generic medicines are those that are copied from branded medicines and give similar results, but they cost less. The April 2020 study by the Confederation of Indian Industry (CII) and KPMG has revealed that 90 percent of the drug market in the US is from generic drugs.
Each of the three consumed medicines is produced by an Indian manufacturer. India receives about 68 percent of its raw material from China, known as Active Pharmaceutical Ingredients (API).
This article is only information/reference purpose and it is only based on recent studies published. This is not substituted for advice from doctors and health professionals. Kindly consult with your doctor before taking any medicine.